This is my TAKHZYRO
IMAGINE yours
Dennis
Real TAKHZYRO patient
Join the more than 3,250 people since 2018* who have chosen to help prevent hereditary angioedema (HAE) attacks before they happen.
*Based on third-party US specialty pharmacy data.
What best describes your experience?
- See all options
-
- See all options
- I'm learning about TAKHZYRO
- I'm taking TAKHZYRO
- I'm caring for someone with HAE
Real TAKHZYRO patient
dennis Real TAKHZYRO patient
Long-term prevention starts here
TAKHZYRO was evaluated in one of the largest prevention studies in HAE.
Explore Study ResultsSELECT IMPORTANT SAFETY INFORMATION
TAKHZYRO may cause serious side effects, including allergic reactions.
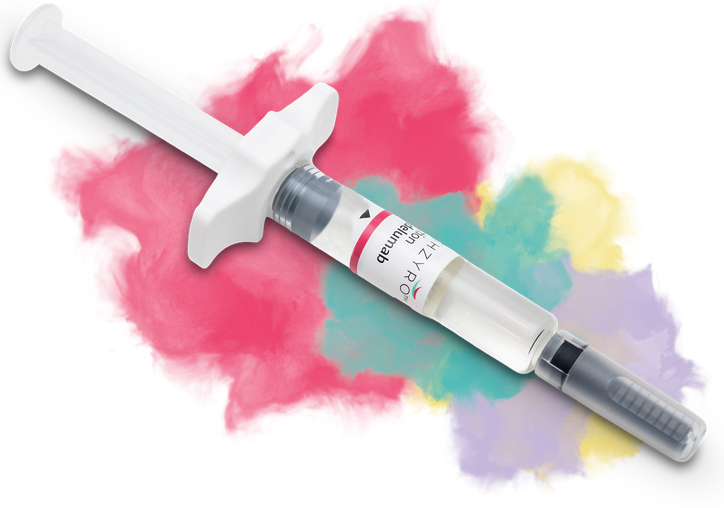
Freedom from daily dosing
TAKHZYRO is a preventive treatment for HAE attacks that adults and adolescents can take just once every 2 weeks. It comes in a single-dose, ready-to-use, prefilled syringe.
The recommended dosage for people 12 years of age and older who are starting on TAKHZYRO is 300 mg every 2 weeks. If you have zero attacks for more than 6 months, your doctor may consider prescribing TAKHZYRO 300 mg every 4 weeks. Do not attempt to take TAKHZYRO without first being trained by a healthcare provider.
The recommended dosage in pediatric patients 6 to less than 12 years of age is 150 mg every 2 weeks. If you have zero attacks for more than 6 months, your doctor may consider prescribing TAKHZYRO 150 mg every 4 weeks.
The recommended dosage in pediatric patients 2 to less than 6 years of age is 150 mg every four weeks.
See the stories of other people living with HAE and taking TAKHZYRO.
soraya Real TAKHZYRO patient
We’ve got your back
Whether you're new to TAKHZYRO or you've been taking it for a while, we have educational resources to help you manage your HAE and stay on track.
Get Tips & Tools
Looking for an easy way to remember your
next TAKHZYRO dose?
Support for everyone
Whether you're helping a loved one find the right treatment or caring for a child with HAE, we're here to support you with information and inspiration.
DOWNLOAD PEDIATRIC BROCHURE Explore Family ResourcesDo you or your loved one need help administering TAKHZYRO?
You should not attempt to administer TAKHZYRO without first being trained by a healthcare provider.
Real patients, real stories
View Transcript
DENNIS: My doctor told me about TAKHZYRO, and my wife and I did some research. At the end of the day, I trusted my medical team and what they told me. After discussing the potential risks and the possibility of fewer HAE attacks, we decided it was the right treatment to take. And it's definitely made a big difference for me. I'm glad I listened.
KELLY: Seeing the clinical data and how effective TAKHZYRO was got my attention. It told me that this is a clinically proven treatment to help prevent HAE attacks, and that gave me the confidence to start.
JACK: For me, taking TAKHZYRO once every two weeks means it's not something I need to think about often. Plus, it's subcutaneous, which means it's injected under the skin, not in the vein. That feels manageable for me.
ANDREW: The actual injection takes about a minute, and then I'm free from thinking about my next dose for a few weeks.
SORAYA: I can inject myself, and it takes about a minute. It's an important thing I can do for myself to help reduce the frequency and severity of my HAE attacks.
NARRATOR: What is TAKHZYRO? TAKHZYRO (lanadelumab) is a prescription medicine used to prevent attacks of hereditary angioedema, HAE, in people 2 years of age and older. It is not known if TAKHZYRO is safe and effective in children under 2 years of age. Important safety information. TAKHZYRO may cause serious side effects, including allergic reactions. Call your healthcare provider or get emergency help right away if you have any of the following symptoms: wheezing, difficulty breathing, chest tightness, fast heartbeat, faintness, rash, hives.
NARRATOR: The most common side effects seen with TAKHZYRO were injection site reactions (pain, redness, and bruising), upper respiratory infection, headache, rash, dizziness, diarrhea, and muscle aches. These are not all the possible side effects of TAKHZYRO. For more information, ask your healthcare provider or pharmacist. You may report side effects to FDA at 1-800-FDA-1088. TAKHZYRO has not been studied in pregnant or breastfeeding women. Talk to your healthcare provider about the risk of taking TAKHZYRO if you are pregnant, plan to be pregnant, are breastfeeding, or plan to breastfeed.
NARRATOR: Please see full Prescribing Information, including information for patients, at TAKHZYRO.com. Talk to your healthcare provider about TAKHZYRO, the only preventive HAE treatment you take as a subcutaneous injection just once every two weeks.
A CLOSER LOOK
AT HAE ATTACKS
Did you know a routine activity
like using scissors can trigger
an
HAE attack?
TAKHZYRO INFO
Find everything you need to
better understand and navigate
TAKHZYRO treatment.
Want to know more about TAKHZYRO and HAE?
Sign up to receive emails and/or text messages with the latest TAKHZYRO news, plus information and resources to help you learn more about HAE and how to manage it.

Personalized product support every step of the way
OnePath® helps people who have been prescribed TAKHZYRO per the approved indication get access to their medication.

