Starting off right with TAKHZYRO

Establishing treatment expectations and goals
HAE is a genetic, unpredictable, and lifelong condition, and it's important to set specific goals for therapy.1
Choosing effective prevention with TAKHZYRO means working together with your patients to help prevent and reduce the severity of their HAE attacks—which may align with their treatment goals.1
Create a regular check-in schedule to review progress and treatment goals—as routine monitoring is recommended by the 2020 HAEA guidelines.1
Soraya
Real TAKHZYRO patient
What does your patient hope to achieve with TAKHZYRO?
Ask your patient to describe their current experience with HAE. This will give you a snapshot of their “before TAKHZYRO.” After your patient has taken TAKHZYRO for a few months, you can compare how the frequency and severity of their HAE attacks may have changed. Have your patient write down the answers to these questions:
- How often are your patient’s attacks now?
- How severe are they?
- How often does your patient use their on-demand medication?
For a comprehensive list of questions of the impact of HAE on your patients, download this Hereditary Angioedema (HAE) Questionnaire for Patients to fill out an initial assessment.
Checking in with patients as they begin treatment
Help adult and adolescent patients ≥12 years of age stay focused on taking TAKHZYRO as prescribed
TAKHZYRO has a half-life of ~14 days and dosing is every 2 weeks. Because of this, it takes ~10 weeks (ie, 6 doses) to reach steady state and ~2 weeks until 50% of TAKHZYRO is eliminated from the body.2,3 This provides patients with freedom from daily dosing.
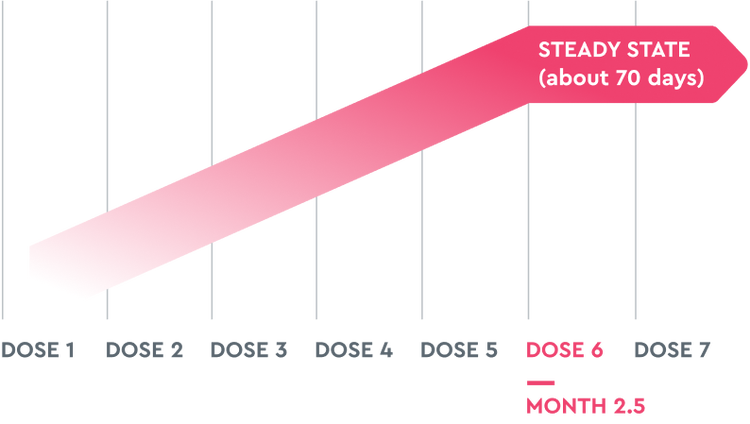
- Continue to check in with your patients even if they have been taking TAKHZYRO for 6 months or longer
- TAKHZYRO has the option for less frequent dosing if a patient is attack free for more than 6 months2
- Remind patients of the impact effective prevention has had on their lives and their progress since starting TAKHZYRO
- Patients taking TAKHZYRO in a 6.5-month study and a 2.5-year open-label extension study had HAE attacks less often. Some patients in the studies had zero attacks for periods of time2,4
Challenge your patient to make a commitment to themselves and TAKHZYRO
Take each dose as prescribed
TAKHZYRO continues to work as it is taken, so it is important to tell your patients to take each dose as prescribed. Remember to impress upon them to avoid skipping or missing doses.2
Breakthrough attacks
It is normal for patients to experience breakthrough attacks.2
It is recommended that patients keep their acute medication on hand in case of a breakthrough attack.1
Let your patient know that if a breakthrough attack happens, they should not be discouraged. They should keep taking TAKHZYRO as prescribed and discuss their experience with you.
Stay the course
Over time, your patient may begin to notice that their attacks are less severe or that they are using their on-demand treatment less. These are signs that TAKHZYRO is working.
Help patients stay prepared to prevent HAE attacks
Encourage your patients to keep taking TAKHZYRO exactly as you prescribed. Although some patients may start to see results quickly, due to a half-life of ~14 days, it takes ~10 weeks (ie, 6 doses) to reach steady state.2,3 Suggest these tips to help your patient initiate a routine and remember their dosing schedule:
- Get alerts or reminders:
- Create a calendar event on their phone or computer with a notification alert that repeats every other week
- Sign up for the TAKHZYRO Text Reminder Program. They will get a text message the day before and the day of their scheduled dose. Tell them to visit TAKHZYRO.com or text SIGNUP (o RECORDAR en español) to 36395 to get started*
- Ask their loved ones for help. Ask a family member or friend to remind them when it’s time to take their TAKHZYRO
- Choose a dosing day that works best for their lifestyle. Think of something that will be easy to remember. For example, they could try "TAKHZYRO Tuesday"
*Message and data rates may apply. Average of 3 messages per month. Text HELP to 36395 for more information or text STOP to 36395 to end text reminders. View our Terms & Conditions at engagedrx.com/tak
Support for your patients backed by Takeda’s 16+ years of experience
What your patients can expect when getting their TAKHZYRO
All HAE medications are provided by specialty pharmacies. Insurance companies often require additional information to approve this type of prescription medication. Sometimes it can take a month to coordinate the delivery of your patients’ first shipment of TAKHZYRO. This is to be expected.
The good news is, we provide support at no cost. And we will work to make sure your patients get their TAKHZYRO as quickly as possible.
Ready to start your patients on TAKHZYRO?
References: 1. Busse PJ, Christiansen SC, Riedl MA, et al. J Allergy Clin Immunol Pract. 2021;9(1):132-150.e3. doi:10.1016/j.jaip.2020.08.046 2. Takhzyro. Prescribing information. Dyax Corp; 2025. 3. Wang Y, Marier JF, Kassir N, Chang C, Martin P. Clin Transl Sci. 2020:13(6):1208-1216. doi: 10.1111/cts.12806 4. Banerji A, Bernstein JA, Johnston DT, et al; HELP OLE Investigators. Allergy. 2022;77(3):979-990. do:10.1111/all.15011