Rediscover effective prevention
The safety and efficacy of TAKHZYRO (lanadelumab-flyo) were assessed in a 6.5 months pivotal trial of 125 HAE patients ≥12 years of age. The primary efficacy endpoint was the rate of investigator-confirmed attacks during the treatment period compared to placebo.1,4
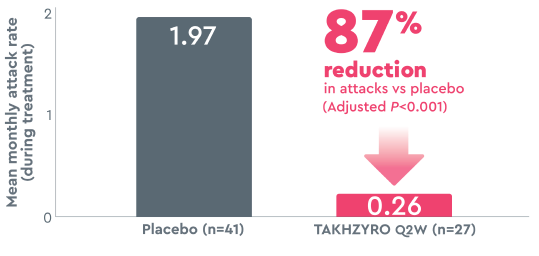
Significant reduction in mean attack rate‡ vs placebo at 6.5 months in the HELP study1,4
- TAKHZYRO 300 mg every 4 weeks resulted in a 73% reduction in attacks vs placebo (Adjusted P<0.001)1§
Mean monthly attack rate during the run-in period4:
| 3.52 for Q2W arm (n=27) |
3.71 for Q4W arm (n=29) |
4.02 for placebo arm (n=41) |
Mean monthly attack rate during the treatment period1:
| 0.26 for Q2W arm |
0.53 for Q4W arm |
1.97 for placebo arm |
All data presented are for TAKHZYRO 300 mg every 2 weeks unless otherwise indicated.1
‡Mean monthly attack rate: number of attacks/4 weeks.1
§Adjusted P-values for multiple testing.1
Q2W=every 2 weeks; Q4W=every 4 weeks.
HELP study: A multicenter, double-blind, parallel group, placebo-controlled clinical study assessed the efficacy and safety of TAKHZYRO in 125 HAE Type I/II patients (≥12 years of age) for 6.5 months. Patients were treated with TAKHZYRO 150 mg q4wks, TAKHZYRO 300 mg q2wks, TAKHZYRO 300 mg q4wks, or placebo. Primary endpoint: rate of investigator-confirmed attacks during the treatment.1,4
The 6.5-month HELP clinical trial of 125 patients led to the 2018 FDA approval of TAKHZYRO
STUDIED IN OVER
800
PATIENTS GLOBALLY3
ACROSS 15 STUDIES
STUDIED FOR
11
YEARS IN US CLINICAL AND REAL-WORLD STUDIES3
PRESCRIBED TO OVER
4000
US PATIENTS SINCE 20183‖
||Based on third-party US specialty
pharmacy data.
Help your patients get started and stay on track with TAKHZYRO.
References: 1. Takhzyro. Prescribing information. Dyax Corp; 2023. 2. Banerji A, Bernstein JA, Johnston DT, et al; HELP OLE Investigators. Allergy. 2022;77(3):979-990. doi:10.1111/all.15011 3. Data on File, TAKHZYRO DOF, Takeda Pharmaceuticals USA, Inc. 4. Banerji A, Riedl MA, Bernstein JA, et al. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773
