HAE IS AN UNPREDICTABLE AND POTENTIALLY LIFE-THREATENING GENETIC DISEASE1
Hereditary angioedema (HAE) is a rare genetic disease that causes recurrent, debilitating, and potentially life-threatening attacks of angioedema in the body. HAE affects about 1 in 50,000 people of all ages.2,3
An accurate and early diagnosis is an important first step in developing an effective management plan for your patients with HAE. Untreated HAE attacks can grow in intensity and may take longer to resolve.3-5
HAE laryngeal attacks can be life-threatening, and they are especially dangerous for children who lack the ability to self-administer treatment during an attack or who may be unable to describe their symptoms.4,5
TAKHZYRO is not indicated for acute treatment.
For both adult and pediatric patients, attacks can occur in the…4
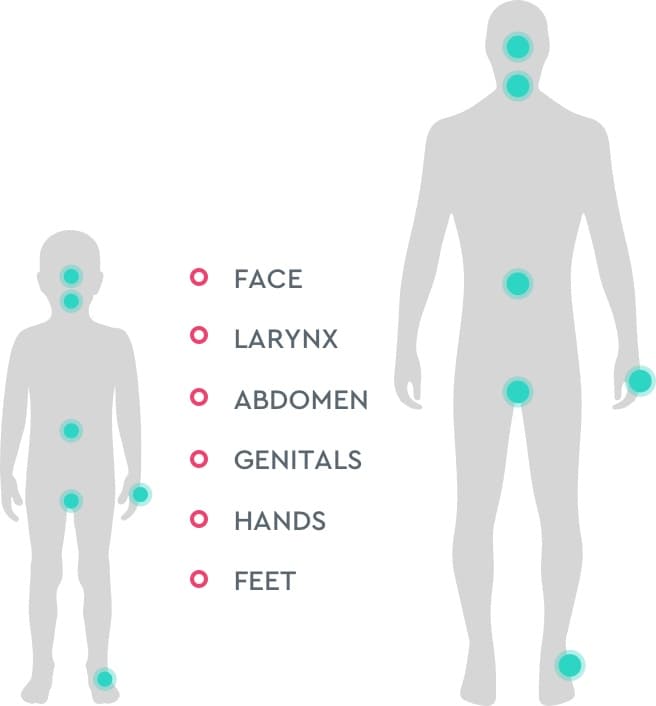
The frequency and severity of HAE attacks may
vary for each individual over time regardless of
age, meaning that past attacks do not predict
the severity of future attacks.7
To help keep track of changes in HAE attacks,
use this Hereditary Angioedema (HAE)
Assessment Questionnaire for Patients.
Since most attacks are unpredictable and not prompted by triggers, the 2021 international WAO/EAACI guidelines suggest physicians should not support excessive avoidance of suspected triggers, which can limit a patient's normal life.8
- High HAE disease activity often comes with impact on daily life8
- The daily lives of some patients with low attack rates are also impacted, thought to be linked to the unpredictability and continuous fear of HAE attacks8
EAACI=European Academy of Allergy and Clinical Immunology; WAO=World Allergy Organization.
Ask yourself the following questions to help understand the signs of a patient who is trigger avoidant with a low attack history
- Does your patient experience HAE swells in their extremities, but not classify them as attacks?
- Does your patient avoid activities because of their HAE?
- Would your patient live their life differently if they were on a preventive treatment?
Explore the impact that TAKHZYRO may offer your patients, including the latest clinical data.
Long-term HAE prevention
should be individualized
and considered in all
patients with HAE8

